ProTarget Cohorts
ProTarget aims to investigate the efficacy and safety of each study drug outside the EMA-approved label and based on genomic matching. To reach this goal, patients are enrolled in cohorts defined by the study drug, the identified actionable, genomic alteration and the tumor type (cancer). The below table lists all cohorts opened in the trial within each study drug. Cohorts marked in green have been evaluated and expanded from stage 1 (8 pts) to stage 2 (24 pts.). Cohorts marked in red are closed and therefore no longer available for inclusion. Cohort monitoring is further described below the table.
Cohort expanded
Cohort closed
Open Study drugs
| Study drug | Variant/cancer (cohort no.) | ||
|---|---|---|---|
| Atezolizumab | TMBhigh | MSIhigh | |
| TMBhigh/prostate (001) | MSIhigh/gastric (010) | ||
| TMBhigh/intestinal (003) | MSIhigh/NEC (038) | ||
| TMBhigh/CRC (006) | MSIhigh/GBM (103) | ||
| TMBhigh/SCLC (007) | MSIhigh/prostate (120) | ||
| TMBhigh/CUP (018) | MSIhigh/CUP (145) | ||
| TMBhigh/breast, non‐TNBC (023) | MSIhigh/pancreas (149) | ||
| TMBhigh/bile ducts & gallbladder (041) | MSIhigh/germ cell tumor (154) | ||
| TMBhigh/NEC (042) | MSIhigh/ovarian (156) | ||
| TMBhigh/ovarian (044) | |||
| TMBhigh/GBM (051) | |||
| TMBhigh/sarcoma (064) | |||
| TMBhigh/penile (071) | |||
| TMBhigh/NET (077) | |||
| TMBhigh/GEJ (109) | |||
| TMBhigh/salivary gland (142) | |||
| TMBhigh/grade III glioma (157) | |||
| Pemigatinib | FGFR | FGF | PDGFRA |
| FGFRfusion/CUP (059) | FGFampl/breast (061) | PDGFRAmut/GBM (104) | |
| FGFRfusion/GBM (067) | FGFampl/prostate (070) | PDGFRAmut/breast (119) | |
| FGFRfusion/grade II glioma (076) | FGFampl/head & neck (096) | ||
| FGFRfusion/NSCLC (083) | FGFampl/NET (140) | ||
| FGFRfusion/bladder & urinary tract (111) | |||
| FGFRfusion/ovarian (112) | |||
| FGFRfusion/intestinal (146) | |||
| FGFRfusion/GEJ (151) | |||
| FGFRmut/SCLC (060) | |||
| FGFRmut/NSCLC (065) | |||
| FGFRmut/bladder & urinary tract (066) | |||
| FGFRmut/esophagus (072) | |||
| FGFRmut/thymic (079) | |||
| FGFRmut/grade III glioma (147) | |||
| FGFRampl/breast (075) | |||
| FGFRampl/esophagus (085) | |||
| FGFRampl/prostate (090) | |||
| FGFRampl/NSCLC (091) | |||
| FGFRampl/endometrial (144) | |||
| FGFRampl/ovarian (158) | |||
| Trastuzumab & pertuzumab | ERBB2 | ||
| ERBB2ampl/bile duct & gallbladder (005) | ERBB2mut/bladder & urinary tract (015) | ||
| ERBB2ampl/intestinal (011) | ERBB2mut/NEC (084) | ||
| ERBB2ampl/salivary gland (019) | ERBB2mut/CRC (128) | ||
| ERBB2ampl/NSCLC (022) | |||
| ERBB2ampl/bladder & urinary tract (027) | |||
| ERBB2ampl/CRC (028) | |||
| ERBB2ampl/esophagus (053) | |||
| ERBB2ampl/endometrial (132) | |||
| ERBB2ampl/head & neck (143) | |||
| ERBB2ampl/ovarian (148) | |||
| Trastuzumab emtansine | ERBB2 | ||
| ERBB2ampl/NSCLC (013) | ERBB2mut/NSCLC (017) | ||
| ERBB2ampl/esophagus (074) | ERBB2mut/head & neck (055) | ||
| ERBB2ampl/bile duct and gallbladder (082) | ERBB2mut/breast (062) | ||
| ERBB2ampl/bladder & urinary tract (094) | ERBB2mut/endometrial (081) | ||
| ERBB2ampl/pancreas (100) | ERBB2mut/cervix (092) | ||
| ERBB2ampl/penile (102) | ERBB2mut/bladder & urinary tract (108) | ||
| ERBB2ampl/HCC (114) | ERBB2mut/SCLC (127) | ||
| ERBB2ampl/CRC (121) | ERBB2mut/CUP (134) | ||
| ERBB2ampl/head & neck (133) | ERBB2mut/CRC (139) | ||
| ERBB2ampl/prostate (141) | ERBB2mut/NEC (155) | ||
| Vemurafenib & cobimetinib | BRAF | ||
| BRAF/NSCLC (002) | BRAF/NEC (029) | ||
| BRAF/thyroid (009) | BRAF/breast (043) | ||
| BRAF/bile duct & gallbladder (012) | BRAF/ovarian (046) | ||
| BRAF/pancreas (014) | BRAF/intestinal (106) | ||
| BRAF/GBM (021) | BRAF/grade III glioma (125) | ||
| Vismodegib | PTCH1 | ||
| PTCH1/head & neck (063) | PTCH1/melanoma (118) | ||
| PTCH1/NSCLC (069) | PTCH1/ovarian (135) | ||
Abbreviations
Ampl.: amplification; CRC: colorectal cancer; CUP: carcinoma of unknown primary origin; GBM: glioblastoma; GEJ: gastro‐ esophageal juction; mut: mutation; NEC: neuroendocrine carcinoma; NET: neuroendocrine tumor; NSCLC: non‐small cell lung cancer; RCC: renal cell carcinoma; SCLC: small cell lung cancer; TNBC: triple negative breast cancer.
Discontinued study drugs
The following study drugs has been discontinued due to completed patient enrollment:
Avelumab
Axitinib
Niraparib
Cohort monitoring
Each cohort is defined by the study drug, tumor genomic alteration and tumor type, and monitored according to a Simon-like two-stage monitoring plan. In first stage, 8 pts. are enrolled in the cohort and response is evaluated (figure 1). If at least one patient demonstrates benefit of treatment, the cohort is expanded with an additional 16 pts. to reach 24 pts. (stage 2), otherwise the cohort will be closed.
A positive signal of drug activity in stage 2 may be concluded if at least 5 pts. among the 24 pts. demonstrate benefit of treatment. If less than 5 pts. demonstrate benefit of treatment, the cohort will be closed.
A positive signal of drug activity may lead to further investigations possibly in collaboration with other European DRUP-like trials.
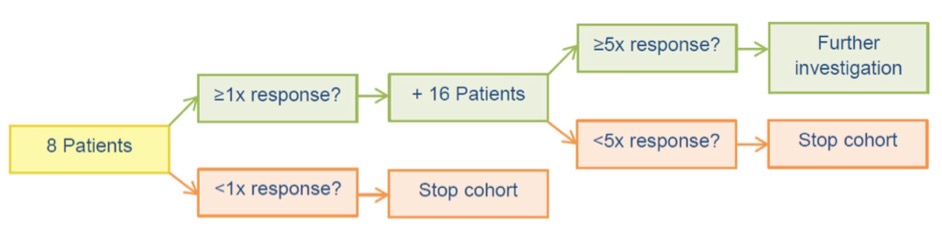
Figure 1. Cohort accrual and monitoring in ProTarget.
